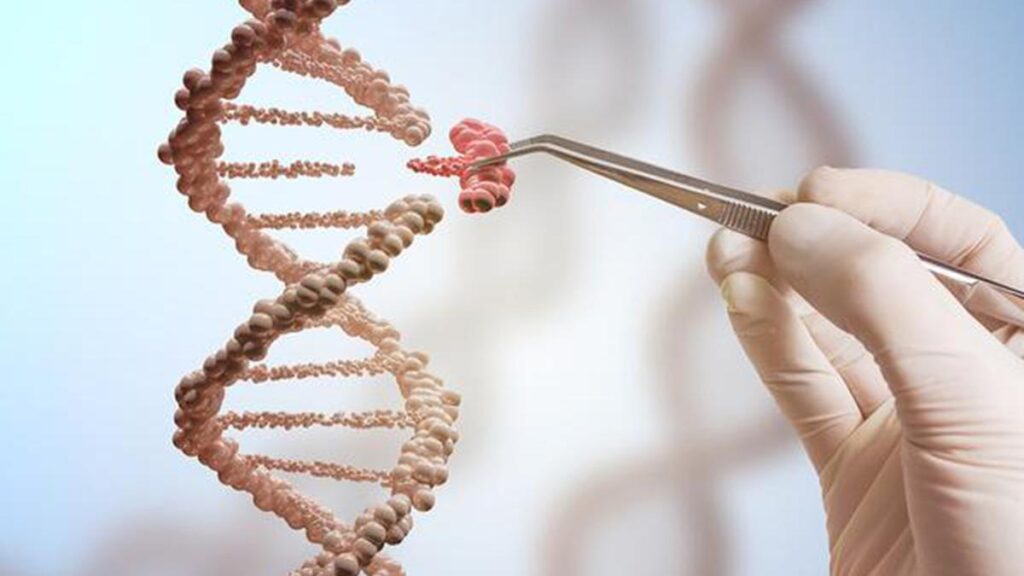
The fourth industrial revolution marked by the fusion of applied sciences is blurring the traces between the bodily, digital and organic worlds. And it’s at this intersection that the way forward for medication is being written, with improvements like CRISPR and CAR-T remedy unlocking the potential to not solely deal with illnesses however treatment them. From modifying the human genome to reprogramming immune cells to struggle most cancers, these approaches promise to rework healthcare as we all know it. However as we stand on the point of this new period, the query is not if these applied sciences will change lives — however how they may reshape the framework of human biology, ethics, and society itself.
In 2012, Jennifer Doudna and Emmanuelle Charpentier revealed their groundbreaking work, revealing that CRISPR/Cas9 might be re-engineered as a gene-editing instrument. They won the Nobel Prize in Chemistry for this, in 2020.
On December 8, 2023, america’ Meals and Drug Administration (FDA) approved of a gene therapy treatment for transfusion dependent beta-thalassemia and sickle cell anaemia (SCA) sufferers — a choice that can revolutionise medication and alter numerous lives.
But, I discover myself grappling with combined feelings. Whereas know-how and medication have all the time been intertwined, by no means have we imagined respiratory life into extinct creatures or mending/tailoring our genes, concepts nothing in need of science fiction.
What’s CRISPR?
Casgevy and Lyfgenia, the 2 cell-based gene therapies authorized by the FDA, utilise the CRISPR/Cas9 genome modifying know-how. Clustered Recurrently Interspaced Brief Palindromic Repeats (CRISPR) /CRISPR-associated protein 9 (Cas 9) advanced naturally as a defence mechanism in micro organism and archaea. It was first reported in E. Coli in 1987 by Ishino et.al.
In a nutshell, the system serves as a genetic reminiscence for previous infections by incorporating part of the viral genetic materials into its personal, in order that the subsequent time it’s invaded, the micro organism is able to recognising the virus and destroying it. The micro organism, in brief, has developed an immunisation mechanism to keep off undesirable viral invaders.
Watch:2020 Chemistry Nobel for developing CRISPR/Cas9 genetic scissors
The CRISPR system is simple to govern. Researchers have tailored it as a instrument to chop, delete, or add DNA sequences at exact areas opening doorways to treating genetic problems, in diagnostics, creating illness/drought resistant vegetation, or in de-extinction initiatives involving the woolly mammoth and the dodo.
The progress in CRISPR has been fast. In solely a decade since its discovery, we now have a know-how with the potential to rewrite genetic code. As thrilling because the prospect is, conversations round moral, societal and questions of safety should progress parallelly.
Moral concerns
A siren rang throughout the scientific world in 2018, when Chinese scientist He Jiankui introduced he had altered a gene in three human embryos to render resistance to HIV. He was imprisoned for three years in 2019 by a court docket in China. The scientific group additionally condemned his actions, and this self-regulation was essential because it was a transparent violation of tips that banned germline modifying. However nonetheless “designer infants” grew to become a actuality. The only real objective of Jiankui and his group experimenting with the embryos was to observe the evolution of two infants, because the genetic intervention was totally different for every embryo. The concept of such instruments within the arms of scientists like Jiankui is terrifying. In an article, Françoise Baylis, a bioethicist at Dalhousie College in Nova Scotia, rightly identified “There’s a distinction between making individuals higher and making higher individuals.”
There’s additionally concern amongst the general public that the rich will exploit this know-how for genetic enhancement. Whereas CRISPR, proper now, is getting used to deal with monogenic problems, it’s able to altering a number of genes. Admittedly nonetheless, elements like intelligence are complicated traits influenced by many genes and the surroundings, making it troublesome to tailor. As well as, CRISPR has additionally bought its personal security issues, resembling off-targeting, that aren’t totally understood but.
Whereas there’s a consensus throughout the scientific group to make use of CRISPR for therapeutic functions, incidents just like the Chinese language case are all the time potentialities.
And so, when advantages outweigh the dangers the place will scientists draw the road? Is it moral to carry out germline modifying, even for the aim of treating illnesses? Relating to human embryos, we face quite a few unresolved ethical questions and have a lot to study what is correct and flawed on this quickly evolving subject.
Additionally Learn:A reckless experiment: on gene edited babies
For a lot of within the disabled group, their genetic anomalies are a part of their identities. This raises vital questions concerning the intersection of ableism and CRISPR, notably concerning the potential implications of societal attitudes in direction of incapacity as genetic applied sciences change into widespread. Whereas most would select gene therapies, there’s a concern that such rising applied sciences might reinforce the notion that incapacity is an abnormality relatively than a pure facet of human variety. This makes it vital to incorporate disabled voices in discussions from the start, previous to medical functions, and on the policy-making degree.
The associated fee issue
One other issue is the exorbitant value of such applied sciences. Casgevy prices round $2.2 million. Sickle Cell Anaemia (SCA) is generally seen in sure ethnic teams together with individuals of African, Mediterranean and Center Japanese descent. In India, SCA is relatively higher in tribal communities. Many individuals from these teams are economically deprived and discover it troublesome to afford even main healthcare. Indian researchers are working in direction of constructing a cheap therapy with CRISPR. However we’re speaking about an enormous leap from fundamental – hydroxyurea – first line therapy in India to CRISPR, a complicated instrument requiring expert consultants. With CRISPR available in the market, it’s of paramount significance to first provoke dialogues on well being fairness.
In line with elementary trigger principle, well being disparities persist as a result of developments in medication have a tendency to profit the extra advantaged segments of society, leaving deprived teams with much less entry to new interventions. As seen throughout occasions just like the COVID-19 pandemic, these with increased social standing — who’ve extra assets — can entry healthcare extra readily than minority populations, who usually face boundaries in acquiring related advantages.
Will CRISPR assist farmers, or will it assist the agribusiness giants that spend money on it? Will it discover a approach to attain the susceptible or will it change into a instrument for the rich? And do we actually want de-extinction initiatives after we are failing to guard extant animals? As we transfer into the longer term and CRISPR turns into simpler to make use of and is best understood, how will future generations select to change human embryos on which at present there’s a moratorium?
The questions are quite a few and the solutions blurry. It’s nonetheless early to say when CRISPR might be inexpensive to a bigger part of individuals. As these applied sciences emerge and evolve at a fast pace, regulatory our bodies should set up strict tips for moral, societal, security and environmental points on the similar tempo.
CAR-T Cell Remedy
The identical 12 months that CRISPR’s use as a gene-editing instrument was found, a six-year-old’s battle with acute lymphoblastic leukaemia (ALL) would outline your complete subject of CAR-T cell remedy. Chimeric Antigen Receptor (CAR) T cell remedy genetically alters a affected person’s T cells (a kind of white blood cell) to struggle malignant tumour cells by focusing on a protein on the floor of most cancers cells.
When the experimental therapy was in its earliest days, a physician, Stephen Grupp, was approached by Emily Whitehead’s mother and father on the lookout for a miracle to avoid wasting their daughter as different medical doctors had given up all hope. The brand-new remedy started its section I medical trial and Emily was enrolled. She grew to become the primary paediatric affected person to obtain the therapy and the primary ever affected person of any age to obtain it for ALL.
Twelve years later, it’s clear the Whiteheads discovered the miracle they sought: Emily stays cancer-free. Nevertheless, the journey was removed from simple. Even medical doctors have been unsure of the therapy’s end result, studying alongside Emily, as challenges arose all through her care.
In 2017, the FDA authorized the primary CAR-T remedy, paving the way in which for a whole lot of sufferers to obtain the therapy, thanks in-part to brave households like Emily’s. It is very important acknowledge although that not all outcomes are as comfortable as Emily’s. Some sufferers stay unresponsive or might relapse. Every case is exclusive and there’s a studying curve with every affected person.
How CAR-T remedy works
T cells possess receptors on their floor that may recognise antigens — proteins or molecules recognized by the immune system — and when overseas antigens are detected, the immune system alerts the T cells to destroy them.
Nevertheless, most cancers cells can typically categorical antigens that the physique doesn’t recognise as irregular, stopping the immune system from sending T cells to assault them. In different cases, even when T cells are current, they is probably not efficient at eliminating most cancers cells.
CAR-T cells are genetically engineered in a laboratory to incorporate a brand new receptor that permits them to bind to and kill most cancers cells. The event of CAR-T cells includes a multi-step course of that begins with accumulating T-cells from the affected person by way of a process referred to as leukapheresis. These T-cells are then modified within the lab to specific CARs on their floor. The gene encoding the CAR is synthesised within the lab, and a vector — usually a viral vector, is used to ship the CAR gene into the T-cells.
As soon as re-engineered, the T-cells are multiplied within the lab to generate hundreds of thousands of cells, that are then despatched again to the hospital for infusion into the affected person. Usually, sufferers bear chemotherapy earlier than receiving the CAR-T cells. Several types of most cancers have distinctive antigens, which signifies that every CAR-T cell remedy is designed to focus on a particular most cancers antigen.

CAR-T cell remedy prices anyplace between ₹3 to 4 crore, excluding hospitalisation expenses, rendering it unavailable to most individuals. Whereas just a few insurance coverage do cowl the bills of the therapy in america, some procedures won’t be lined. Logistics, journey and meals are different bills one wants to bear in mind whereas present process this therapy.
The unaffordability of CAR-T remedy fuelled Rahul Purwar’s vision of growing an indigenous model. At present a professor on the Indian Institute of Bombay (IIT-B), Dr. Purwar returned to India in 2013 after finishing his postdoctoral programme at Harvard Medical Faculty. Alongside together with his analysis college students — Alka Dwivedi, Atharva Karulkar — and haemato-oncologists Gaurav Narula and Hasmukh Jain from Tata Memorial Hospital, it took a decade to convey this imaginative and prescient to fruition.
The method of designing CAR-T cells requires experience. The researchers collaborated with consultants on the Nationwide Most cancers Institute (NCI) to beat the challenges they confronted. The group needed to then strategy the Central Medication Commonplace Management Organisation (CDSCO) for medical trial approval, which concerned a number of rounds of revisions. It was additionally a primary for CDSCO, as tips weren’t framed with respect to cell remedy, and so they advanced together with the know-how.
In October 2023, India bought its first indigenous CAR-T remedy — NEXCAR19 — that prices round ₹45 lakh, a fraction of its U.S. counterpart. This sum, nonetheless, nonetheless stays unaffordable for a majority of Indians, for whom accessing even main healthcare is troublesome. Proximity to a well-equipped hospital can also be vital, as some instances might require intensive care utilisation after the therapy and follow-ups frequently.

And in order with CRISPR, we circle again to the query of well being fairness with rising medical applied sciences. Can we hope the costs will lower as soon as manufacturing will increase? Can these applied sciences be carried out in sub-urban and rural India?
There isn’t a doubt that CRISPR and CAR-T cell remedy are breakthroughs within the medical subject. However as a result of they’re nonetheless of their preliminary phases, they arrive with a million-dollar price ticket. Creating new therapies is dear. Along with the price of analysis, manufacturing, labour, logistics, advertising, distribution and mental property improvement, comes the added work of regulatory approval.
However what does the longer term maintain for such rising traits that interweave totally different fields to pave the way in which for enhancing individuals’s lives, if the outcomes are however accessible to a handful?
(Soujanya Padikkal is a contract content material supplier based mostly in Hyderabad)
Printed – February 24, 2025 11:41 am IST