 Sanjeev Panchal, Nation President & Managing Director for AstraZeneca India; Picture: Selvaprakash Lakshmanan for Forbes India
Sanjeev Panchal, Nation President & Managing Director for AstraZeneca India; Picture: Selvaprakash Lakshmanan for Forbes India
AstraZeneca has had a powerful presence within the Indian marketplace for almost 45 years, introducing over 50 progressive medicines so far. In India, the pharma main’s focus stays on areas together with biopharmaceuticals (comprising cardiovascular, renal metabolism and respiratory), oncology, immune therapies and uncommon illness. “We wish to deliver progressive medicines to India, sooner,” says Sanjeev Panchal, nation president and managing director, AstraZeneca India. At this time, the corporate is extra dedicated than ever to accelerating the event and supply of breakthrough therapies, with a spotlight to fast-track its progressive drug pipeline in India.
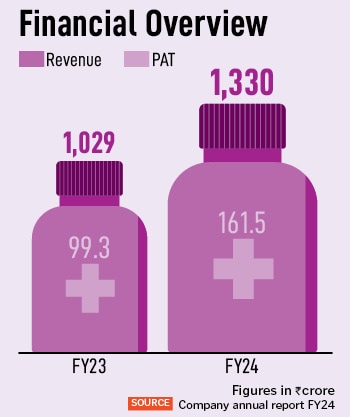
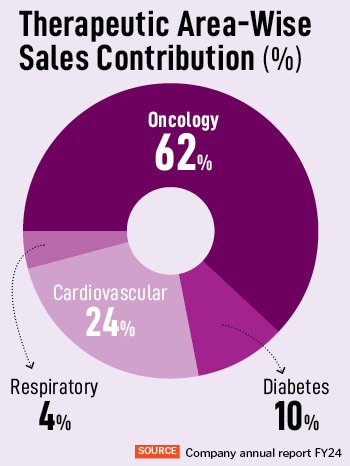 AstraZeneca India introduced its Q2 FY24-25 outcomes at the moment, touching a 31 p.c enhance in income from operations in comparison with the identical interval final 12 months. For Q2 FY24, its income from operations is Rs 408 crore. The corporate has achieved a year-on-year development of 29 p.c, with Rs 1,330 crore in revenues in FY24. “We’re strengthening our footprint in India, for India and the world,” says Panchal, who has been with the organisation for near 21 years, in numerous roles and throughout geographies. Of this, oncology accounts for 62 p.c of the corporate’s whole income, and has additionally seen the very best income development of 43 p.c. “These key remedy areas are pivotal for our development, by way of the innovation technique,” provides Panchal.
AstraZeneca India introduced its Q2 FY24-25 outcomes at the moment, touching a 31 p.c enhance in income from operations in comparison with the identical interval final 12 months. For Q2 FY24, its income from operations is Rs 408 crore. The corporate has achieved a year-on-year development of 29 p.c, with Rs 1,330 crore in revenues in FY24. “We’re strengthening our footprint in India, for India and the world,” says Panchal, who has been with the organisation for near 21 years, in numerous roles and throughout geographies. Of this, oncology accounts for 62 p.c of the corporate’s whole income, and has additionally seen the very best income development of 43 p.c. “These key remedy areas are pivotal for our development, by way of the innovation technique,” provides Panchal.
In November 2023, AstraZeneca Pharma India Ltd introduced its exit from manufacturing at its plant in Bengaluru, as a part of a worldwide strategic overview. “This resolution was additionally made in keeping with our deal with specialist illness areas, and our efforts to deliver medicines sooner to India through imports,” he provides. AstraZeneca India has determined to import specialist medicines to India, and concurrently companion regionally to develop entry the place applicable.
Specializing in innovation
AstraZeneca’s mission is to make most cancers as manageable as diabetes. “We’re main a revolution in oncology to redefine most cancers care. We’re following the science to know most cancers and all its complexities to find, develop and ship life-changing remedies and enhance the potential to remedy,” says Anil Kukreja, former vp, medical affairs, AstraZeneca Pharma India.
Also read: Global pharma: Focus on core business, pure-play innovation
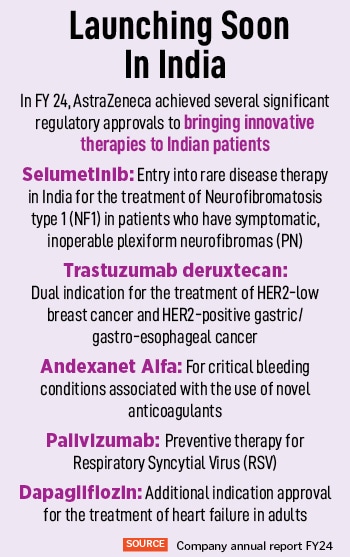
With oncology, the corporate has medicines focussed on various kinds of most cancers, together with lung most cancers, breast most cancers and gastrointestinal (GI) most cancers. “We’re additionally increasing, when it comes to indications. As an example, earlier we had been late-stage lung most cancers, however now we’re increasing to new medicines or indications that can be utilized in early levels,” says Panchal. Earlier this 12 months, the corporate launched Trastuzumab deruxtecan, a remedy tailor-made for the remedy of grownup sufferers with unresectable or metastatic HER2 optimistic breast most cancers for individuals who have beforehand obtained an anti-HER2 routine.
 Inside oncology, the pharma big is engaged on antibody drug conjugates (ADC), tumour drivers and resistance therapies, and immuno-oncology (IO). “ADCs are extremely focused and have a cell-specific mechanism to cut back uncomfortable side effects for these being handled. We’re additionally growing the subsequent wave of IO therapies that purpose to empower the immune system, to extra successfully recognise and kill most cancers cells and to beat the immunosuppressive mechanisms that cancers steadily develop as they evolve,” explains Kukreja.
Inside oncology, the pharma big is engaged on antibody drug conjugates (ADC), tumour drivers and resistance therapies, and immuno-oncology (IO). “ADCs are extremely focused and have a cell-specific mechanism to cut back uncomfortable side effects for these being handled. We’re additionally growing the subsequent wave of IO therapies that purpose to empower the immune system, to extra successfully recognise and kill most cancers cells and to beat the immunosuppressive mechanisms that cancers steadily develop as they evolve,” explains Kukreja.
For biopharmaceuticals, AstraZeneca India has obtained approvals for important remedies resembling a triple inhalation aerosol for managing persistent obstructive pulmonary illness (COPD) delivered by way of a tool, Palivizumab, for stopping respiratory syncytial virus (RSV) for untimely and high-risk infants, and Andexanet Alfa for managing life-threatening bleeding linked to Issue Xa (FXa) inhibitors. “I believe what’s vital for me is the unmet want within the illness areas and the place we will take advantage of significant distinction to sufferers,” says Panchal.
On the immunology entrance, AstraZeneca’s Synagis (Palivizumab), a monoclonal antibody, has been permitted and just lately launched in India for the prophylaxis of decrease respiratory tract illness (LRTD) attributable to respiratory syncytial virus (RSV) in high-risk youngsters like pre-terms (Kids born at 35 weeks of gestation or much less), Kids lower than 2 years of age and with bronchopulmonary dysplasia and congenital coronary heart illness.
The technique for AstraZeneca India is to recognise the unmet wants in illness areas, and to take advantage of significant distinction to sufferers. Panchal provides, “As an example, uncommon illnesses. We introduced a drugs for Neurofibromatosis sort 1 [NF1] final 12 months. We wish to proceed specializing in such gaps,” he remarks. With this in thoughts, the corporate is concurrently taking part in additional international scientific trials throughout its remedy areas.
“From a topline perspective AstraZeneca India is doing effectively; they’re the quickest rising pharma MNC in India, clocking a 30 p.c topline development,” says Vishal Manchanda, senior vp, Institutional Analysis, Systematix Group.
The goal, based on Panchal is to the touch 15 new launches—a mix of latest indications and medicines—by 2025. “We wish to be main in specialist illnesses and really rework affected person outcomes, by specializing in early prognosis. And of those 15, we already have 9 new approvals, so we’re on observe.”
Accessibility and affordability
Whereas AstraZeneca India has been bringing many progressive medicines to India, the problem of accessibility and affordability, stays. To resolve for this, the corporate is working with numerous state governments in addition to many Indian gamers to succeed in the plenty. Panchal says, “Because the world is transferring in direction of focused remedy for most cancers, we’re prioritising work round early detection of lung most cancers, breast most cancers and GI most cancers.”
As an example, the corporate has collaborated with Qure.ai, an organisation growing deep studying algorithms for radiology picture interpretation for lung most cancers. “About 85 p.c of lung most cancers instances are identified late. It is because when the nodule is small within the lungs, the affected person has no signs. By the point, signs are seen it’s too late and there may be solely a 5 p.c survival for late-stage detection of lung most cancers,” explains Prashant Warier, co-founder and CEO, Qure.ai. Normally, lung most cancers is detected through focused CT screenings. However usually the difficulty is that not sufficient folks get these screenings or there isn’t sufficient capability to accommodate folks particularly for the free screenings. “However with Qure.ai’s resolution, docs can display screen sufferers for lung most cancers by way of X-rays, guaranteeing that at-risk sufferers achieve entry to mandatory therapies sooner,” provides Warier.
A Tripartite settlement between AstraZeneca Pharma India Pvt Ltd with help of Karnataka authorities, Qure.ai has deployed this resolution in 19 district hospitals throughout the state. Together with AstraZeneca India, now we have now scaled as much as greater than 30 international locations and the corporate has dedicated to processing 5 million scans by way of Qure.ai’s know-how by 2025,” says Warier.
Lately, the corporate signed a memorandum of understanding (MoU) with the Authorities of Goa to speed up lung most cancers detection, emphasising early detection and intervention in combating lung illnesses. The corporate additionally signed an MoU with Rajiv Gandhi Most cancers Institute and Analysis Centre (RGCI&RC), Delhi, to ascertain a centre of excellence for subsidised, high-quality next-generation sequencing (NGS) molecular panel testing for people identified with lung most cancers. Moreover, the pharma firm has partnered with Roche Diagnostics India to reinforce diagnostics for breast most cancers sufferers, specializing in streamlining HER2 diagnostics with developments within the subject.
 Aside from innovator medicine, AstraZeneca additionally has a bunch of medicine that cater to the plenty. For these, AstraZeneca India entered into an settlement with Mankind Pharma Restricted for unique distribution of its bronchial asthma drug model Symbicort (budesonide and formoterol fumarate dihydrate), an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) mixture, in India. “Whereas we deal with specialist medicines, we additionally imagine there are some medicine that want to succeed in a better variety of sufferers. Therefore, partnering with a participant like Mankind expands entry,” says Panchal. The partnership goals to speed up entry and maximise the potential of the bronchial asthma drug and the Turbuhaler, a tool to ship a better proportion of respirable particles.
Aside from innovator medicine, AstraZeneca additionally has a bunch of medicine that cater to the plenty. For these, AstraZeneca India entered into an settlement with Mankind Pharma Restricted for unique distribution of its bronchial asthma drug model Symbicort (budesonide and formoterol fumarate dihydrate), an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) mixture, in India. “Whereas we deal with specialist medicines, we additionally imagine there are some medicine that want to succeed in a better variety of sufferers. Therefore, partnering with a participant like Mankind expands entry,” says Panchal. The partnership goals to speed up entry and maximise the potential of the bronchial asthma drug and the Turbuhaler, a tool to ship a better proportion of respirable particles.
“Most MNCs have been following this pattern—this partnership is sensible as a result of AstraZeneca has restricted bandwidth in India, and may hardly do justice to a product like Symbicort, which is a mass-market product. It could want a big gross sales drive to be efficient. Therefore, it’s applicable to leverage Mankind’s attain for such merchandise; it finally ends up being a win-win for each,” explains Manchanda.
The Indian pharmaceutical trade should prioritise innovation to stay aggressive and meet evolving well being care wants. Lately, the federal government launched an R&D coverage to encourage native innovation. “We try to make our medicines extra reasonably priced for sufferers. On this additionally, the federal government introduced GST discount. Lastly, a scientific trial waiver was additionally introduced, beneath which if a drug is permitted in six international locations, there can be a scientific trial waiver. So, whereas there are challenges, we’re seeing progress, which provides the worldwide firm confidence to speculate extra in a market like India,” explains Panchal.
AstraZeneca’s tech play
The corporate has two entities, one in all which is listed—AstraZeneca Pharma India Restricted—and the opposite AstraZeneca India Non-public Restricted, which caters to IT providers, international enterprise providers and R&D capabilities.
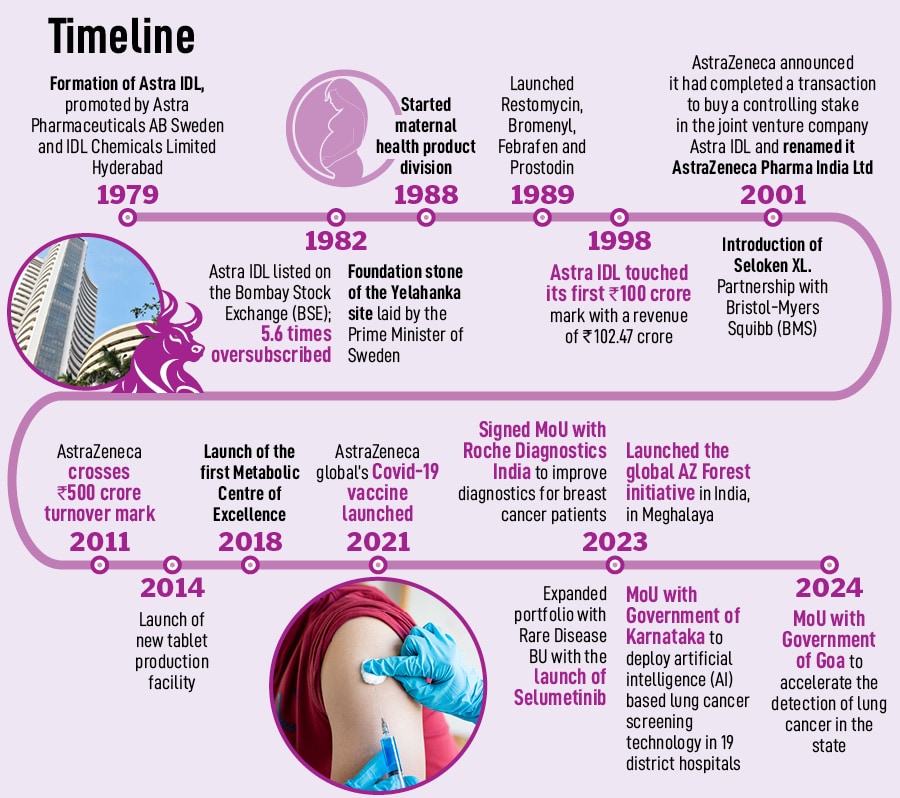
Earlier this 12 months, AstraZeneca India introduced its plans to speculate Rs 250 crore to develop its World Innovation and Know-how Centre (GITC) in India, creating round 1,300 extremely expert roles by 2025. “India has grow to be AstraZeneca India’s largest footprint for know-how on the planet,” provides Panchal.
When AstraZeneca established the GITC in Chennai in 2014, it was principally an IT help providers centre with 300 folks, however now it has developed into a worldwide hub of innovation. “In alignment with our mission of utilizing science to enhance sufferers’ lives, we leverage knowledge analytics, synthetic intelligence and machine studying to drive transformation in well being care,” remarks Panchal. India is the nerve centre of know-how and innovation for AstraZeneca, main the corporate’s digital agenda worldwide.
As AstraZeneca India celebrates 45 years of its presence in India, Panchal reckons, “Our goal is to push the boundaries of science, to ship life-changing drugs. We wish to deliver the science to the affected person, and rework the way forward for well being care.”
