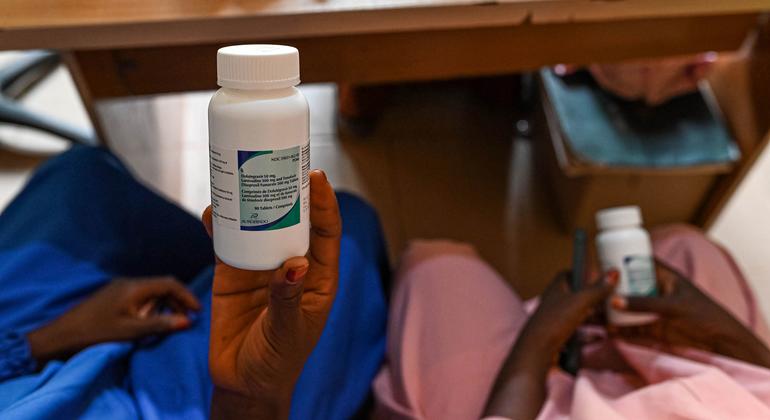
The event marks a milestone for a area that bears almost 65 per cent of the worldwide HIV burden and has lengthy trusted imports of lifesaving antiretroviral medicines and testing kits. However which may be beginning to change.
The human immunodeficiency virus (HIV) weakens the physique’s immune system, decreasing its capacity to battle infections and sure cancers. With out well timed intervention, it will probably progress to acquired immunodeficiency syndrome (AIDS), essentially the most superior stage of an infection.
In 2023, Kenya-based pharmaceutical firm Common Company Ltd grew to become the primary African producer to obtain World Well being Group (WHO) prequalification to provide tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD) – a first-line antiretroviral remedy for HIV.
Now, in a serious step ahead, the World Fund – a worldwide partnership financing HIV, tuberculosis and malaria responses – is procuring this domestically produced HIV remedy for Mozambique, making it the primary time African-manufactured TLD has been deployed by means of this channel.
“The procurement of the African-manufactured first-line HIV remedy by the World Fund for Mozambique is a superb milestone in direction of strengthening provide chain programs in Africa,” said Meg Doherty, Director of WHO’s World HIV Programmes.
“It will contribute to raised well being outcomes for individuals residing with HIV who want uninterrupted medication provides.”
Constructing regional capability
WHO says the achievement is a part of a broader push to bolster native manufacturing capability and enhance entry to important well being applied sciences throughout Africa.
The UN company has been partnering with nations, producers and international well being organizations – together with the World Fund and Unitaid – to increase quality-assured African manufacturing.
“Native manufacturing of quality-assured well being merchandise is an pressing precedence,” stated Rogerio Gaspar, WHO Director for Regulation and Prequalification.
“With each African producer that meets WHO prequalification requirements, we transfer nearer to a extra self-reliant, resilient and equitable well being system.”
Progress, however structural gaps stay
Regardless of the milestone, WHO cautioned that manufacturing alone is just not sufficient. To make sure long-term sustainability, the company is looking for superior market commitments, truthful procurement insurance policies and ongoing technical assist.
WHO additionally factors to diagnostics as a important hole. With shifting donor funding, many nations are below strain to take care of HIV testing programmes, that are the frontline of prevention and remedy.
In a associated effort, Codix Bio, a Nigerian diagnostics firm, not too long ago received a sublicense to manufacture rapid diagnostic tests for HIV.
Domestically produced HIV fast exams will assist improve affordability, and handle provide chain vulnerabilities and delays
– WHO Director Meg Doherty
“Having domestically produced HIV fast exams will assist improve affordability, and extra broadly handle provide chain vulnerabilities and delays in entry to diagnostics,” stated Dr. Doherty.
Sustaining affect amid funding pressure
As a part of its steerage, the UN well being company can be encouraging nations to undertake low-cost, WHO-prequalified fast HIV exams, particularly as the primary take a look at in nationwide algorithms, which may considerably lower prices whereas sustaining service supply.
Whereas the newest replace marks tangible progress, extra motion is required.
“Domestically manufactured TLD is a serious step in direction of that purpose,” WHO stated, “however extra motion is required.”