In September 2022, former President Biden convened the White Home Convention on Starvation, Diet, and Well being, throughout which the White Home launched its Nationwide Technique on Diet and Well being (National Strategy). The Nationwide Technique known as for creating extra accessible meals labeling practices to empower customers to make more healthy selections, amongst different laudable public health-focused targets. Previous to the January 2025 transition from the Biden to the Trump administration, the Meals and Drug Administration (FDA) took concrete steps to deal with this explicit Nationwide Technique precedence by each formal rulemaking and casual steering. This weblog put up summarizes FDA’s actions on the finish of the Biden administration supposed to modernize meals labeling practices and transfer them ahead in right now’s extra consumer-focused market.
Proposed Rule for Entrance-of-Package deal Diet Labeling
Within the Nationwide Technique, the event of front-of-package (FOP) labeling schemes was mentioned as one technique to promote equitable entry to diet data and more healthy selections. On January 16, 2025, FDA revealed within the Federal Register a proposed rule that will require a front-of-package diet label on packaged meals (Proposed Rule). The Proposed Rule would require producers so as to add a “Diet Information” field on the principal show panel of every packaged meals product, which might record the Every day Worth (DV) proportion of saturated fats, sodium, and added sugars in a serving of that meals. The DV proportion would record how a lot of the nutrient in a serving contributes to an individual’s whole every day weight loss program. As well as, every of these vitamins would come with corresponding “interpretative data” that will sign to customers whether or not the meals product incorporates a low, medium, or excessive quantity of these vitamins. An instance of the proposed FOP diet data graphic is under. And though the Proposed Rule wouldn’t require it, producers might voluntarily embody a calorie depend on the entrance of the meals bundle, per current FDA laws.
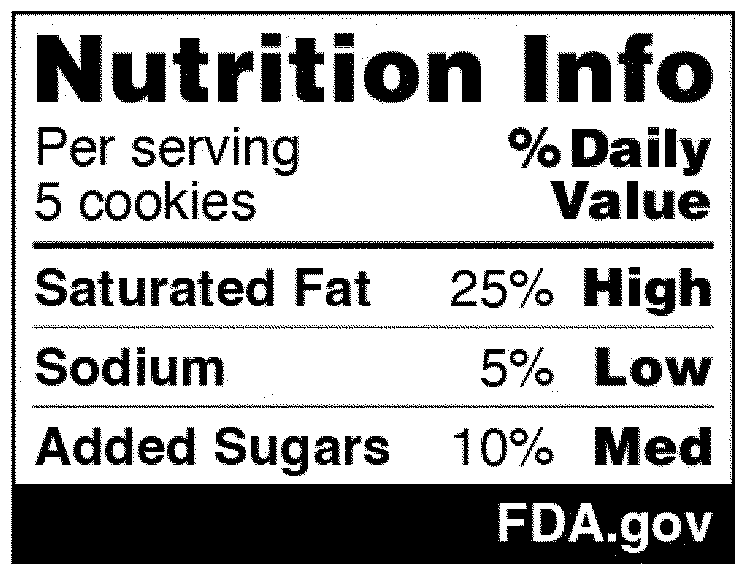
The Proposed Rule does deviate from sure recommendations made within the Nationwide Technique, which advocated for FOP “star scores” and “site visitors mild schemes” to advertise equitable entry to diet data. Particularly, the Nationwide Technique thought-about find out how to finest assist customers with decrease diet literacy extra readily establish meals that comprise a nutritious diet. As a substitute of a front-of-packaging labeling system that will depend on imagery, nonetheless, FDA’s proposal opted for written details about the vitamins contained within the meals. Each the preamble to the Proposed Rule and FDA’s press release saying its publication clarify that in focus teams carried out in 2022, individuals reported confusion over the site visitors mild system specifically (e.g., when a meals contained each vitamins that must be restricted but additionally vitamins for which greater consumption is beneficial) and that “the black and white Diet Information scheme with the p.c [DV] carried out finest in serving to customers establish more healthy meals choices.”
It is going to be fascinating to see whether or not feedback to the Proposed Rule will comment on FDA’s selection of the written “Diet Information” field versus a FOP labeling system that will be extra reliant on imagery. FDA is accepting feedback on the Proposed Rule till Might 16, 2025 (Docket FDA-2024-N-2910). As presently envisioned, if the proposal for FOP diet data is adopted, most meals product producers would have three years from the efficient date to deliver labels into compliance (smaller producers can be given 4 years).
On account of President Trump’s administrative freeze and new government orders governing the work of regulatory companies resembling FDA, the destiny of this Proposed Rule is presently unsure. Nevertheless, newly confirmed Well being and Human Providers (HHS) Secretary Robert F. Kennedy Jr. has articulated that his agenda is to “make America wholesome once more” (MAHA) and the presidential MAHA Commission was recently established to start informing the Administration’s work on Mr. Kennedy and President Trump’s priorities on this area. Though Mr. Kennedy didn’t handle meals labeling throughout his Senate affirmation hearings and the manager order creating the MAHA Fee doesn’t converse on to meals labeling or diet data accessibility for customers, stakeholders ought to monitor the upcoming work of the Fee – together with whether or not any alternatives for public feedback could also be made obtainable – in addition to its future “Make Our Youngsters Wholesome Once more Technique” that’s due in roughly six months. Additional, beneath a deregulatory executive order signed on January 31, 2025, President Trump has directed companies to eradicate 10 “laws” for every new regulation to be promulgated, with the time period “regulation” expansively outlined to incorporate memoranda, steering paperwork, coverage statements, and interagency agreements. This “one-in, 10-out” order might make the prospect of an FOP diet labeling closing rule much less seemingly, not less than for the foreseeable future.
Remaining Rule for Use of The Time period “Wholesome” on Meals Labeling
One other current FDA motion associated to meals labeling was the company’s finalization of a proposed rule from 2022 that concerned a prolonged public session and knowledge assortment course of (see our prior protection here). On December 27, 2024, FDA revealed within the Federal Register its Final Rule concerning the usage of the time period “wholesome” in meals labeling. The Remaining Rule updates the definition established 30 years in the past for the nutrient content material declare “wholesome” for use in meals labeling. In President Biden’s Nationwide Technique, one highlighted precedence was guaranteeing that meals packages bearing this declare align with present diet science and the Dietary Guidelines for Americans (Dietary Pointers). To advance this aim, FDA was charged with updating the requirements for when an organization can use the “wholesome” declare on its merchandise (work on which was already ongoing on the company), creating a logo that can be utilized to mirror that the meals is “wholesome, and creating steering on the usage of Dietary Guideline statements on meals labels.
The unique regulatory definition of “wholesome” (codified at 21 C.F.R. § 101.65(d)) units limits on whole fats, saturated fats, ldl cholesterol, and sodium content material ought to a meals be labeled as wholesome, and requires that the meals comprise not less than 10% of the DV for vitamin A, vitamin C, calcium, iron, protein, and fiber. Below the Remaining Rule, whole fats and dietary ldl cholesterol are now not elements to be thought-about when evaluating whether or not a meals is eligible for this explicit nutrient content material declare. As a substitute, the company has established limits on saturated fats, sodium, and added sugars in accordance with the Dietary Pointers. Moreover, fairly than specializing in vitamin A, vitamin C, calcium, iron, protein, and fiber, the Remaining Rule requires that the meals product comprise a specific amount of meals from not less than one of many meals teams or subgroups beneficial by the Dietary Pointers, resembling fruit, greens, grains, dairy, and proteins.
Maybe most notably, the prior regulatory scheme allowed for meals that had been excessive in added sugars, resembling yogurts, breakfast cereals, and fruit snacks, to technically qualify as “wholesome” regardless of not aligning with the definition of “nutrient-dense” meals from the Dietary Pointers, which particularly applies to sure meals “when ready with no or little added sugars, saturated fats, and sodium.” In line with typically accepted dietary finest practices, the Nationwide Technique additionally promoted reducing the sodium content material in meals and reducing the consumption of added sugars –shared targets of recent HHS Secretary Kennedy and the broader MAHA agenda.
The Remaining Rule doesn’t set up a “wholesome” image that can be utilized on meals packaging, however FDA has indicated that this image might also be on the horizon. In its press release announcing the Final Rule, FDA famous that it’s “persevering with to develop” this image, including that such a logo would additional FDA’s aim of serving to customers extra simply establish more healthy meals merchandise.
The Remaining Rule’s efficient date (which as of publication of this weblog put up, has not been modified by the Trump Administration) is February 25, 2025, and the compliance date for producers is February 25, 2028 – three years after the brand new regulatory definition turns into efficient.
Draft Steerage for Trade: Labeling of Plant-Primarily based Options to Animal-Derived Meals
Lastly, whereas not particularly known as out within the Nationwide Technique, FDA has been working for a number of years to develop labeling suggestions for plant-based meals which can be being developed and marketed as options to standard animal merchandise. On January 7, 2025, FDA launched the Draft Steerage for the Labeling of Plant-Primarily based Options to Animal Derived Meals (Draft Guidance), in response to the rising demand for plant-based meals options in the USA. In keeping with the Plant-Primarily based Meals Affiliation, 70% of Individuals are consuming plant-based meals. The scope of the newly launched steering encompasses options to poultry, meat, seafood, and dairy merchandise that fall beneath FDA’s jurisdiction. It expressly excludes plant-based milk options, as separate guidance on that subject was launched in February 2023.
The Draft Steerage notes that fairly than merely figuring out a product as a “plant-based” various meals, the precise plant supply must be disclosed on the meals product’s label. This could allow customers to make extra knowledgeable selections about buying plant-based options. For instance, fairly than labeling a plant-based cheese solely as such, the cheese’s label ought to extra clearly disclose “soy-based cheese” to mirror its main components. The Draft Steerage additionally recommends that if a plant-based various meals is derived from a number of completely different plant sources, the first plant sources must be recognized within the meals’s title. The company supplies the examples of “Black Bean Mushroom Veggie Patties” and “Chia and Flax Seed Egg-less Scramble” for example this idea. For labeling functions, FDA additionally recommends firms keep away from completely naming merchandise with “vegan,” “meat-free,” or “animal-free.”
Public feedback on the Draft Steerage must be submitted by Might 7, 2025 (Docket FDA-2022-D-1102).
Conclusion
One main aim of the Nationwide Technique was to empower Individuals to make more healthy, knowledgeable selections about their diet and meals consumption. In the USA, diet-related illnesses, resembling hypertension, weight problems, and diabetes, are on the rise. Below the Biden administration and the management of former Commissioner Dr. Robert Califf, FDA sought to battle these alarming developments and to enhance public well being by growing entry to dietary data and selling transparency in meals labeling.
Additional, whereas the Proposed Rule, Remaining Rule, and Draft Steerage all deal with labeling packaged meals merchandise that may be bought in shops, will probably be fascinating to see how these initiatives affect FDA’s suggestions for meals labeling practices in on-line grocery buying. On April 24, 2023, FDA revealed the discover Food Labeling in Online Grocery Shopping; Request for Information (Docket No. FDA-2023-N-0624-0002), which obtained 31 electronically submitted feedback from numerous stakeholders, together with grocer organizations, meals scientists, and particular person customers. Certainly, the December 2024 press launch for the Remaining Rule famous that FDA “has already entered right into a partnership with Instacart to make it even simpler for customers to seek out merchandise with the ‘wholesome’ declare by on-line grocery buying filters and a digital storefront.” Within the wake of the company actions summarized on this put up and the Instacart partnership, we marvel if FDA will transfer sooner or later to supply producers and retailers with definitive steering on on-line meals labeling practices. We will probably be watching to see how FDA, in addition to the work of the MAHA Fee and HHS Secretary Kennedy, might proceed to enhance meals labeling practices sooner or later.
[View source.]
